Medical Writing
We provide you with high-quality documents to ensure successful review by regulators, notified bodies or publishers.
Clinical and regulatory medical writing
The key to a successful regulatory submission lies in the technical expertise of the managing team. Our medical writing specialists possess not only the required medical device regulatory and clinical expertise, but have also acquired years of hands-on clinical experience throughout a multitude of medical and therapeutic areas.
From a regulatory perspective, clinical evaluation plans and reports need to demonstrate relevant clinical evidence in order for a medical device to access or maintain its place on the market. That’s why our experienced regulatory writing professionals deliver effective and compliant documents for submission by manufacturers to their notified bodies.
Having developed hundreds of clinical investigation protocols and reports, we also offer services in both clinical investigation design and documentation writing for pre-market and post-market clinical follow-up of your medical device.
We can help you:
- Define/refine the claims of the product for efficient development of the clinical evaluation process for successful market access or maintenance of CE mark.
- Successfully transit from MDD to MDR.
- Meet the deadlines for CE-mark by careful planning and preparation of the clinical evaluation process.
- Input to practical post-market strategies based on clinical evaluation findings.
- Formulation of a feasible and practical Clinical Development Plan to support the manufacturer’s clinical investigation plans/future global strategies.
- Step-by-step approach, validated with our customers to avoid misinterpretations and ensure efficient workflow.
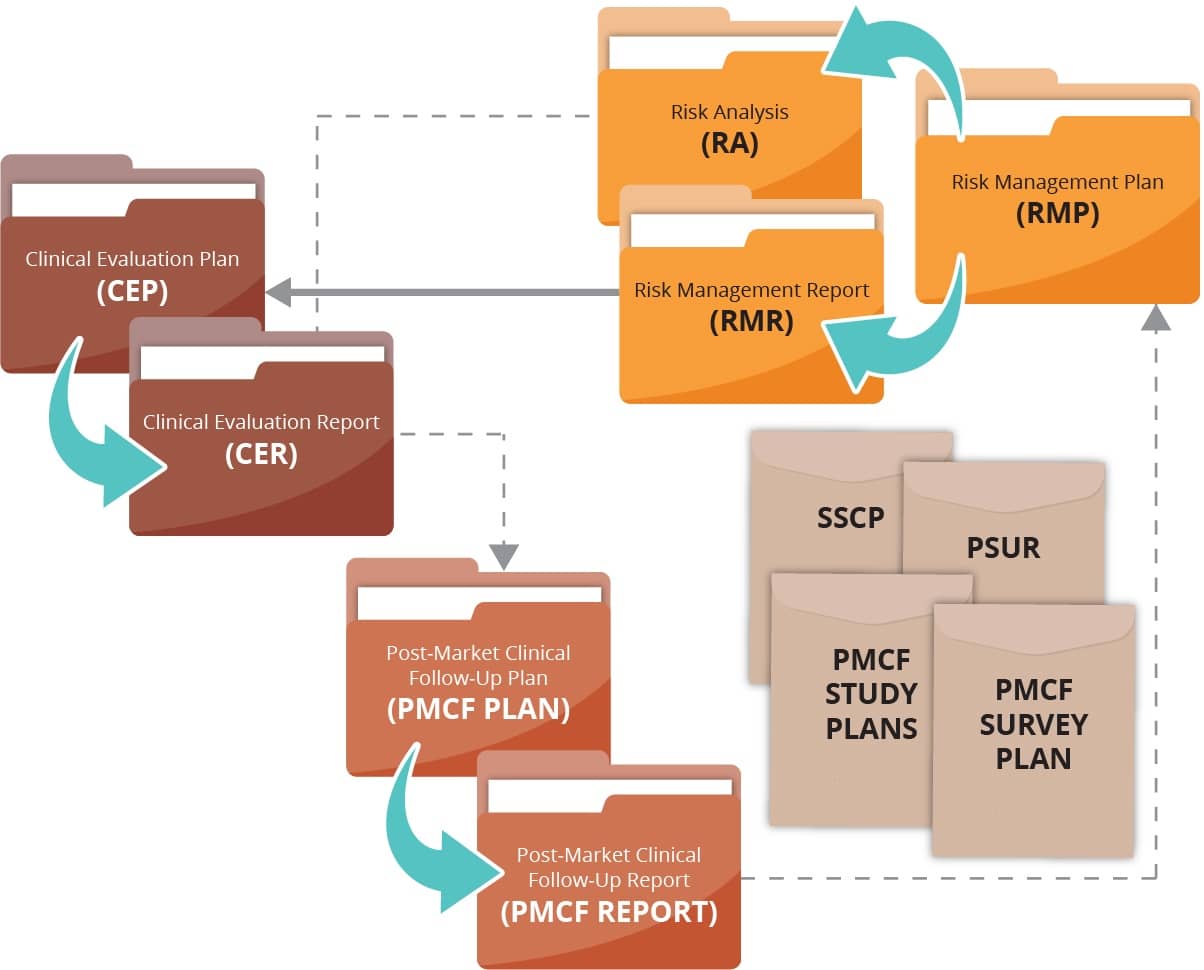
Clinical evidence / clinical evaluation: Do you have the right flow?
We support you with getting the information flow in the right order to produce meaningful and compliant clinical evidence reports.
 Contact us for a free half-hour consultation
Contact us for a free half-hour consultation
